Identifying Polar And Nonpolar Bonds . Electronegativity is a measure of the tendency of an atom. polar covalent bonds. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. it provides examples so you can quickly distinguish nonpolar molecul. Some bonds between different elements are only minimally polar, while others are strongly polar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures.
from www.numerade.com
as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. Some bonds between different elements are only minimally polar, while others are strongly polar. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom. it provides examples so you can quickly distinguish nonpolar molecul. polar covalent bonds.
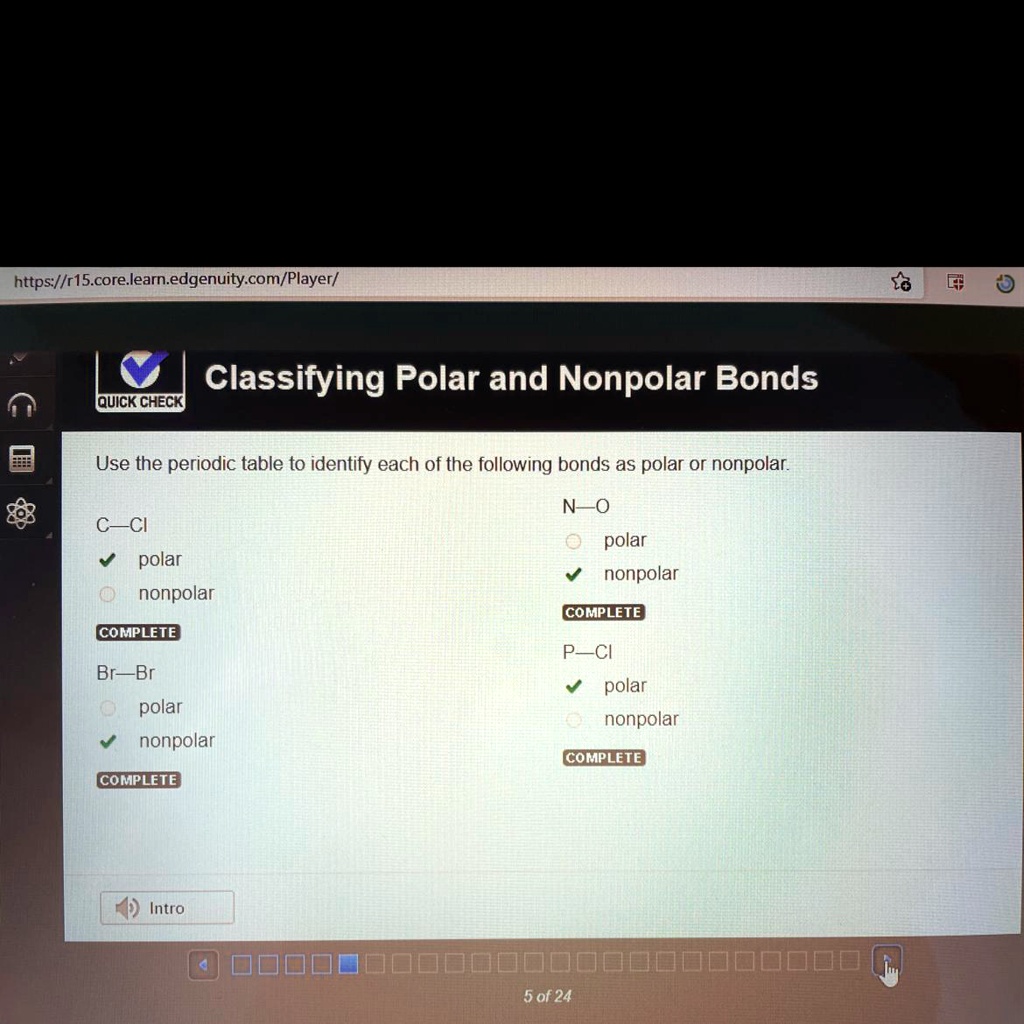
SOLVED 'Use the periodic table to identify each of the following bonds
Identifying Polar And Nonpolar Bonds Electronegativity is a measure of the tendency of an atom. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Electronegativity is a measure of the tendency of an atom. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. polar covalent bonds. Some bonds between different elements are only minimally polar, while others are strongly polar. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. it provides examples so you can quickly distinguish nonpolar molecul. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.
From www.pinterest.com.mx
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry 10 Identifying Polar And Nonpolar Bonds A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. it provides examples so you can quickly distinguish nonpolar molecul. whether a bond is nonpolar or polar. Identifying Polar And Nonpolar Bonds.
From worksheetcampusnicked.z13.web.core.windows.net
Electronegativity Rules For Polarity Identifying Polar And Nonpolar Bonds it provides examples so you can quickly distinguish nonpolar molecul. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Electronegativity is a measure of the tendency of an atom. whether a bond is. Identifying Polar And Nonpolar Bonds.
From aznswerzoneticbacchanals.z21.web.core.windows.net
Polarity Of Bonds Chart Identifying Polar And Nonpolar Bonds polar covalent bonds. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Electronegativity is a measure of the tendency of an atom. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. as a rough guide, bonds. Identifying Polar And Nonpolar Bonds.
From www.youtube.com
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds YouTube Identifying Polar And Nonpolar Bonds to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. Electronegativity is a measure of the tendency of an atom. it provides examples so you can quickly distinguish nonpolar molecul. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent. Identifying Polar And Nonpolar Bonds.
From printableagreeriagedv6.z22.web.core.windows.net
How To Determine Bond Polarity Identifying Polar And Nonpolar Bonds Some bonds between different elements are only minimally polar, while others are strongly polar. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. it provides examples so. Identifying Polar And Nonpolar Bonds.
From chem.libretexts.org
8.7 Bond Polarity and Electronegativity (\(\chi\)) Chemistry LibreTexts Identifying Polar And Nonpolar Bonds it provides examples so you can quickly distinguish nonpolar molecul. Some bonds between different elements are only minimally polar, while others are strongly polar. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Electronegativity is a measure of the tendency of an atom. polar covalent bonds. . Identifying Polar And Nonpolar Bonds.
From sciencenotes.org
Polar and Nonpolar Molecules Identifying Polar And Nonpolar Bonds Electronegativity is a measure of the tendency of an atom. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. whether a bond is nonpolar or polar covalent. Identifying Polar And Nonpolar Bonds.
From www.animalia-life.club
Electronegativity Difference Bond Type Identifying Polar And Nonpolar Bonds it provides examples so you can quickly distinguish nonpolar molecul. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. any covalent bond between atoms. Identifying Polar And Nonpolar Bonds.
From surfguppy.com
What is Nonpolar Covalent Bond Identifying Polar And Nonpolar Bonds Electronegativity is a measure of the tendency of an atom. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. it provides examples so you can quickly distinguish nonpolar molecul.. Identifying Polar And Nonpolar Bonds.
From www.numerade.com
SOLVED Classification Identifying Polar and Nonpolar Covalent Bonds Identifying Polar And Nonpolar Bonds Some bonds between different elements are only minimally polar, while others are strongly polar. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. it provides examples. Identifying Polar And Nonpolar Bonds.
From gambr.co
️Polar Or Nonpolar Molecules Worksheet Free Download Gambr.co Identifying Polar And Nonpolar Bonds whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. as a rough guide, bonds between atoms whose electronegativities. Identifying Polar And Nonpolar Bonds.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID6790720 Identifying Polar And Nonpolar Bonds A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. polar covalent bonds. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. it provides examples so you can quickly distinguish nonpolar molecul. to determine if. Identifying Polar And Nonpolar Bonds.
From learninglicem3d.z21.web.core.windows.net
Identify Types Of Chemical Bonds Identifying Polar And Nonpolar Bonds A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. it provides examples so you can quickly distinguish nonpolar molecul. whether a bond is nonpolar or polar. Identifying Polar And Nonpolar Bonds.
From pediaa.com
Difference Between Polar and Nonpolar Bonds Identifying Polar And Nonpolar Bonds to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. it provides examples so you can quickly distinguish nonpolar molecul. whether a bond is nonpolar or polar covalent is. Identifying Polar And Nonpolar Bonds.
From printablegnusobimab1.z22.web.core.windows.net
Polarity Of Molecules Practice Identifying Polar And Nonpolar Bonds whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. Electronegativity is a measure of the tendency of an atom. A bond in which the electronegativity difference between the atoms is. Identifying Polar And Nonpolar Bonds.
From rhiannond-decree.blogspot.com
Ch4 Polar Or Nonpolar Covalent Bond Covalent bond Simple English Identifying Polar And Nonpolar Bonds A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. polar covalent bonds. any covalent bond between atoms of different elements is a polar bond, but the degree. Identifying Polar And Nonpolar Bonds.
From www.coscinecreative.com
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative Identifying Polar And Nonpolar Bonds any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between atoms whose. whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms. Identifying Polar And Nonpolar Bonds.
From sites.google.com
2.2.2 (i,j) Electronegativity and Bond Polarity Ellesmere OCR A level Identifying Polar And Nonpolar Bonds whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. polar covalent bonds. to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called. Identifying Polar And Nonpolar Bonds.